You could be a
for
Clinically proven to treat all forms of moderate Lumbar Spinal Stenosis.
Schedule your visit today to receive a precise diagnosis and treatment plan from our award-winning physicians!
The Racz Procedure
Minimally Invasive.
Non-Surgical.
Clinical evidence for the Vertiflex™ Procedure shows:
- 90% patient satisfaction five years after treatment
- 66% of patients saw back pain improvement; 75% saw leg pain improvement
- Results showed an 85% decrease in the proportion of patients using opioids between baseline and 5 years post-treatment
Frequently Asked Questions
About Racz
Lumbar spinal stenosis leads to the narrowing of the spinal canal. If left untreated, the narrowing may cause constriction of the nerves that run down the back and into the legs. This can result in pain and discomfort in your back and legs and limit your mobility. For most people, symptoms develop gradually over time and are often made worse by walking or standing. Symptoms of LSS may include:
- Pain while walking
- Numbness or “tingling” feeling in your legs, calves, or buttocks
- Weakness
- Loss of balance
- Aching, dull back pain spreading to your legs
- Decreased endurance during physical activities
- Tendency to sit or lean over slightly to relieve pain
The Vertiflex® Procedure using the Superion® Indirect Decompression System is safe and FDA-approved. It is clinically proven to provide effective long-term relief. It supports your existing anatomy and is completely reversible.
The Vertiflex Procedure was developed to provide patients with a minimally invasive alternative when conservative treatment has failed and other options may be perceived as too aggressive.
The Vertiflex® Procedure is a minimally invasive outpatient treatment. It uses a small implant that relieves pressure on the affected nerves. It was designed with patient safety and comfort in mind. The implant works by supporting your existing anatomy and is reversible, leaving all treatment options available in the future. The Vertiflex Procedure may not be right for everyone, as any treatment has associated risks. Ask your doctor if the Vertiflex Procedure is right for you.
Most incision sites will have a few stitches or staples that should be kept clean and dry until the first follow-up visit, usually 7 to 14 days after having the procedure.
For 6 weeks following your procedure, limit bending and strenuous activity, including lifting anything over 10 pounds.
All patients have different needs. You should always follow your treating physician’s instructions.
The Vertiflex Procedure underwent one of the most rigorous studies on Lumbar Spinal Stenosis. 470 patients were enrolled in an Investigational Device Exemption or IDE trial at 29 sites with a 24-month follow-up and annually thereafter through 60 months.
1. At 5 years, 84% of patients demonstrated clinical success on at least two of three ZCQ domains.
2. Individual ZCQ domain success rates were:
• 75% symptom severity
• 81% physical function
• 90% patient satisfaction
3. Leg and back pain (VAS) success rates were:
• 80% leg pain improvement
• 65% back pain improvement
4. ODI success rate was 65%.
5. 75% of patients were free from re-operation, revision or supplemental fixation at their index level at 5 years.
Five-Year Durability of Stand-Alone Interspinous Process Decompression for Lumbar Spinal Stenosis
Pierce D. Nunley, M.D., Vikas V. Patel, M.D., Douglas G. Orndorff, M.D., William F. Lavelle, M.D., Jon E. Block, Ph.D., Fred H. Geisler, M.D., Ph.D.
Published in Clinical Interventions in Aging, 2017
The Vertiflex Procedure was developed with patient safety and comfort in mind.
Its minimally invasive approach is preferred among many doctors across the United States.
General anesthesia is not required and there is minimal recovery time, getting you home the same day.
The spacer is designed to support your existing anatomy and does not require removal of bone or tissue, making it a safe option for LSS treatment.
The Vertiflex Procedure was the subject of an extremely thorough FDA clinical trial for LSS. Results from the trial proved the effectiveness of the procedure, with significant improvement in pain relief.
The procedure can be completed in an outpatient setting and most patients can return home within the same day.
Vertiflex is covered by Medicare and several private insurance companies, based on medical necessity (Each insurance company and patient’s plan may vary. Please work with your physician to verify benefits and coverage.)
See if Vertiflex is right for you!
When simple activities such as walking or standing become a burden, you may find relief from leg and back pain with the Vertiflex Procedure.
- FDA approved
- Same-day procedure
- Quick recovery time
- Reversible
- Covered by Medicare and most private insurance companies
The procedure can be completed in an outpatient setting and most patients can return home within the same day.
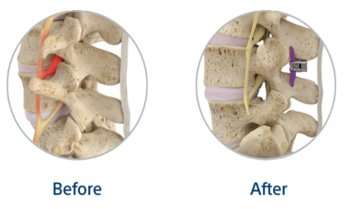
Placement
A small spacer is placed inside the spine without removal of any nearby bone or tissue.
Activation
Once inserted, the spacer’s arms open around the spinous processes (the bumps you can feel in your spine) to help make proper space for the affected nerves.
Relief
The spacer preserves the space in the spine, which keeps pressure off the nerves that cause leg and back pain.
Titanion Alloy Spacer
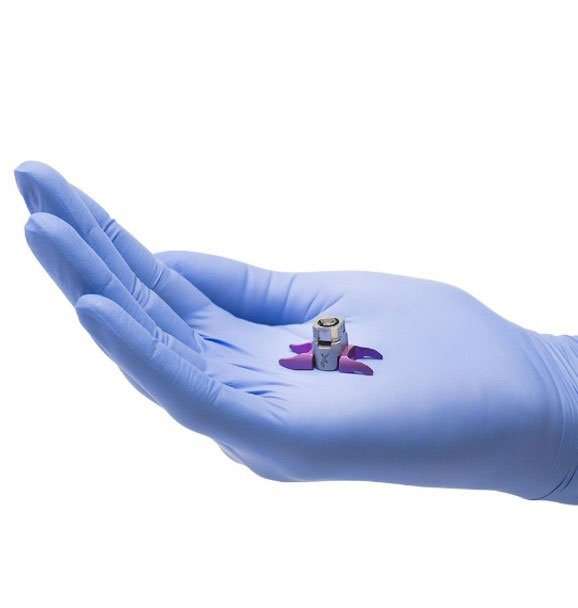
How does the Vertiflex Procedure fit my pain?
The Vertiflex Procedure is intended to provide the same relief you experience when leaning forward or sitting down. It fits your pain by reducing or eliminating the pressure on the nerves caused by LSS. The spacer is also available in several different sizes to fit your anatomy.
Over 20,000 patients have received pain relief from the Vertiflex Procedure since FDA approval.
Proven Results
FDA approved
20K+ proven pain relief
85% opioid reduction
90% patient satisfaction
*Decrease in the proportion of patients using opioids compared to baseline at five years. ‡Study completers.
1. Nunley PD, Deer TR, Benyamin RM, Staats PS, Block JE. Interspinous process decompression is associated with a reduction in opioid analgesia in patients with lumbar spinal stenosis. Pain Res. 2018;11:2943-2948. 2. Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging. 2017;12:1409-1417. N=88.
Real Patient Stories

Procedure

Procedure

Procedure

Procedure

Procedure

Procedure

Procedure

Procedure

Procedure
HAVE A QUESTION?
You deserve guidance and support, we're here to help
- Free 20-minute call with a Care Coordinator
- Ask questions and discuss candidacy
- The fastest way to schedule with a specialist
We'd love to chat
Our team is happy to help with any questions you may have. We are available for calls and live chat during typical business hours, and you can always communicate with us at your convenience.


